검색결과 리스트
글
엔지니어링 플라스틱 Polyketone 합성법
Propagation[edit]
A mechanism for the propagation of this reaction using a palladium(II)-phenanthroline catalyst has been proposed by Brookhart:[7]
Polyketones are noted for having extremely low defects (double ethylene insertions or double carbonyl insertions, in red):
The activation barrier to give double carbonyl insertions is very high, so it does not occur.[6] Brookhart's mechanistic studies show that the concentration of the alkyl-ethylene palladium complex required to give double ethylene insertions is very low at any one point:
Additionally, the Gibbs energy of activation of the alkyl-ethylene insertion is ~ 3 kcal/mol higher than the corresponding activation barrier for the alkyl-carbon monoxide insertion. As a result, defects occur at an extremely low rate (~ 1 part per million).[7] The industrially-relevant palladium-dppp catalyst has also been investigated.[8]
Importance of bidentate ligands[edit]
Where palladium(II) pre-catalysts bearing monodentate phosphine ligands are used in methanol, a relatively high fraction of methyl propionate is produced. In comparison, where chelating diphosphine ligands are used, this side-product is absent. This observation is rationalized: the bis(phosphine) complex can undergo cis-trans isomerization to give the sterically favored trans isomer. The propionyl ligand is now trans- to the open coordination site or ethylene ligand, and is unable to undergo migratory insertion. Instead, solvolysis by methanol occurs, which gives the undesired methyl propionate side-product.[6]
'이론' 카테고리의 다른 글
| 사출성형 이론 (0) | 2014.05.27 |
|---|---|
| 동력학 이란? (0) | 2013.10.01 |
| 폴리카보네이트(PC : polycarbonate) 개요 (1) | 2013.09.23 |
| 엔지니어링 플라스틱(엔프라)의 개요 (0) | 2013.09.23 |
| 친수성 물질이란? (0) | 2013.06.11 |



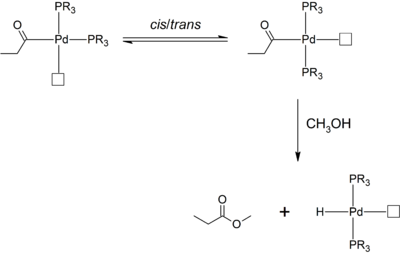
RECENT COMMENT